Thank you for your interest in the Rosenzweig Group. Our research program aims to develop new techniques for the synthesis of 2D and 3D luminescent and electronic nanomaterials. We are focusing primarily on replacing currently used toxic and rare nanomaterials with benign and earth abundant nanomaterials to minimize adverse effects on human health and the environment while reducing the cost of nanotechnology applications. Current research projects in the laboratory involve the synthesis of luminescent quantum dots, luminescent polymer dots, luminescent rare earth containing nanocrystals and 2D materials including but not limited to InSe and MXenes. We are investigating the role the materials’ composition and surface chemistry play in their functional properties, chemical stability, and environmental and human health impact. Of particular interest is the formation of new nanomaterial architectures that combine 2D materials with nanoparticles to form new materials with newly defined functions. We are also investigating the feasibility of using our MXene 2D materials, with or without conjugated luminescent nanoparticles, as delivery vectors in agricultural applications. Finally, we are exploring the use of measurement techniques commonly used in our laboratory in combination with computational techniques (in collaboration with the Bennett’s laboratory) to interrogate works of art to better understand the impact of various adsorbates on their chemical stability and appearance. Research in our laboratory is supported by research grants from NSF, DOE, and the Mellon Foundation.
Research Supported By NSF Award CHE-1904600
InP/ZnS Luminescent Quantum Dots for Bioimaging with Improved Cellular Targeting Capabilities
ABSTRACT
Quantum dots are extremely small nanocrystals of semiconductors that are 100,000 times smaller than a grain of salt and contain just a few thousand atoms. When exposed to light, quantum dots luminesce, and the color of their emitted light depends on their size. It is these unique optical properties that make quantum dots useful in applications ranging from cell phones to light emitting diodes (LEDs). Quantum dots have also attracted the attention of the biotechnology community due to their potential use in bioimaging and biosensing applications. However, most quantum dots contain heavy metals like cadmium and lead that are toxic to living cells and their surfaces quickly become coated with proteins and lipids that degrade their stability. With support from the Macromolecular, Supramolecular and Nanochemistry Program in the Division of Chemistry, Professor Zeev Rosenzweig in the Department of Chemistry and Biochemistry at the University of Maryland Baltimore County (UMBC) is developing non-toxic quantum dots from indium phosphide. Working with his students and collaborator Dr. Karen Leinkamp at Albert-Ludwigs Universitat in Germany, Professor Rosenzweig is modifying the surface of the quantum dots with novel polymers that inhibit the formation of protein corona on their surface, allowing them to maintain their bright luminescence and stability in biological solutions. Their discoveries could have broad implications for using semiconductor quantum dots in biological applications. The project provides interdisciplinary research training opportunities for graduate and undergraduate students involved. Professor Rosenzweig and his team are also actively engaged in outreach to nearby high schools to introduce students to concepts in nanoscience and nanotechnology, including their impact on human health and the environment.
Research Supported by NSF Award CHE-1503408
Center for Sustainable Nanotechnology with Robert Hamers (University of Wisconsin Madison, PI), Zeev Rosenzweig (UMBC, Co-PI), and 14 other Co-PIs
ABSTRACT
The Center for Sustainable Nanotechnology (CSN) enables fundamental studies of the specific molecular interactions expected to occur when the surfaces of engineered nanoparticles come into contact with biological interfaces and components. The chemical insight gained from molecular and mechanistic studies of nano-bio interactions is used to intentionally design nanomaterials that provide the desired technical functions while also minimizing potentially adverse consequences with the environment. CSN research leads to introduction of a new generation of safer nanomaterials that may impact multiple sectors of the economy including energy, transportation, electronics, food, and agriculture. The CSN prepares a new generation of scientific innovators by providing education and professional development opportunities focused on innovation, communication, leadership, and interdisciplinary scientific skills. CSN researchers actively encourage diversity within chemistry through CSN-run workshops on overcoming implicit bias, recruitment of graduate students and postdocs, and Center-sponsored research experiences for students and faculty from underrepresented groups as well as veteran undergraduates. CSN researchers encourage diversity in public engagement, develops and practices informal communication skills, and expands the highly successful sustainable-nano.com blog to include Spanish language content, K-12 activity materials, podcasts, and videos.
CSN integrates experiments with computation in three integrated research focus areas (RFAs). RFA1 develops new materials with precisely controlled structure and properties, molecular-level characterization tools such as advanced non-linear optical methods and sub-diffraction imaging, and novel computational methods spanning length scales from nanometers to millimeters. RFA2 uses the RFA1 tool kit to understand, control, and predict how nanomaterials attach to, penetrate, and alter cell surfaces using lipid bilayers as model systems for cell membranes. RFA3 determines the molecular processes by which pristine and environmentally transformed nanomaterials interact with living systems with diverse cellular chemistries, ranging from single cell to multi-cellular organisms. The integration of these three RFAs enables the Center to establish how nanomaterial properties and behavior impact biological outcomes. This knowledge leads to reliable predictions of biological responses to existing and future nanomaterials and guides the design and synthesis of safe, sustainable nanomaterials. In pursuing the project goals, the CSN puts particular emphasis on the synthesis, characterization, and molecular-level interactions initiated by complex, composite nanomaterials that are being used in emerging and future nano-enabled commercial technologies.
Research Supported by Mellon Foundation Award 41500634
A Baltimore Based Research and Education Consortium at the Interface between Science and Arts (Baltimore SCIART)
ABSTRACT
The objective of this proposed project is to continue and improve a Baltimore-based academia-museum consortium, named (Baltimore-SCIART) to mentor a diverse group of undergraduate students, primarily from the Baltimore region with a specific focus on students who show strong interest in the field of art conservation science, and demonstrated commitment to broadening participation of students from underrepresented groups. Recognizing the increasingly multidisciplinary nature of the field, the consortium will bring together the expertise of eight University of Maryland Baltimore County (UMBC), Johns Hopkins University (JHU), and Morgan State University scientists in the fields of chemistry, physics, materials science and engineering, and a conservation scientist and conservators from the Walters Art Museum, all based in the Baltimore area. Within the consortium, the Walters will continue to offer the unique paired expertise in conservation and conservation science in its department of conservation and technical research, as well as wide-ranging collection materials for study and art historical/curatorial expertise. The Baltimore SCIART consortium will offer 10-week long summer research program for undergraduate students in the field of art conservation science. The proposed program will increase awareness among the participating students of the field of art conservation, and the world of arts. Another important goal of the consortium is to make an impact on the public in Baltimore through the organization of public outreach events at UMBC, the Walters Art Museum and other venues. Another important new program component is the addition of a full-time postdoctoral research fellow who engages in cutting-edge research in art conversation science on research projects of interest to the Walters Art Museum, and the art conservation science community.
Research Supported by DOE Award DE-SC0020346
Expanding the Utility and Range of Quantum and Polymer Dots for Multiplexed Super Resolution Fluorescence Imaging in Plants
ABSTRACT
The overlapping emission spectra of endogenous plant pigments currently limit the number of fluorophores with different emission colors that can be imaged simultaneously and, therefore, the number of cellular components that can be imaged within a single experiment. Hence, developing fluorescent tags and a microscope system that can image beyond the visible range to the near infrared (NIR) would greatly enhance plant imaging capabilities. Extending imaging into the NIR region would support the interrogation of multiple cellular molecular targets simultaneously, but it would inevitably decrease spatial resolution due to the increase of the diffraction limit of light. To address this need, we propose to develop an integrated super resolution fluorescence imaging (SRFI) platform that would allow imaging of both the visible and NIR-emitting molecules, as well as quantum dots (QDs) and polymer dots (Pdots). We will further develop Pdots by encapsulating 2-3 organic dyes emitting in the visible region and the NIR to enable multiplexed imaging using fluorescent barcodes. We will demonstrate the application of the platform to determine the functional roles of putative membrane receptors in plants.
Research Supported by NIH SBRI Award 1R43GM131486-01
SeeTrue: The Microinjection Colored Needle For Enhanced Visibility and Anti-Clogging Capabilities
ABSTRACT
Microinjection is a widely used transgenic engineering technique that underpins genetic manipulation, experimentation, and knowledge discovery across virtually all areas of biomedical science. It consists of using an industry standard, transparent, clear glass microneedle (ISN) to manually inject foreign materials (e.g. RNA and DNA), into biological cells. This thirty-year-old technology has driven advances in the fundamental research of stem-cell gene manipulation, created the intra-cytoplasmic sperm injection method as part of in- vitro fertilization cycle therapy, and allowed extensive human disease prevention modeling via pathophysiological investigations. Three design-induced constraints often attributed to poor user technique – affect the technique’s efficacy and experimental rigor and reproducibility: (1) the transparent needle tip creates low contrast visibility in vitro, leaving the user unable to view and track the penetrating needle tip; (2) cytoplasmic material (e.g. embryo yolk) can clog the needle tip, diminishing volume injected over time; and (3) manual creation of each needle via industry standard pipette pullers introduces calibration variability with every new needle used. Although other foreign-material-introduction methods exist, such as electroporation, chemical membrane permeabilization, and automated, robotic systems, none have proven responsive to addressing these key design constraints. The objective of this proposal, prototyping a colored-tip, microcapillary needle with anti-clogging properties as a minimal viable product (MVP), bridges an important need in the biomedical industry. The working hypothesis is that the patented, SeeTrue MVP will simply and powerfully increase the accuracy of the microinjection technique as compared to ISNs by a) improving the efficacy of delivering material to the embryo, and b) reducing variability in the volume injected. The MVP will be tested, and its efficacy compared to ISN’s in the Zebrafish embryo biological system by both experienced (18+ months) and inexperienced (less than one month) Zebrafish embryo microinjectors. The proposed SeeTrue MVP introduces simple, elegant, design innovations of enhanced visibility and anti-clogging properties to an established, fundamental technique. It has the potential to correct known rigor and reproducibility (technique) deficits and increase the efficacy of microinjection biomedical transgenic research. This will, in turn positively impact foundational human-health related research and medical applications.
Current Projects:
Understanding the Mechanism of Degradation of Red Lake Pigments in Heterogeneous Materials
Lake pigments are organic pigments prepared by precipitating a dye with an inert binder, usually a metallic salt such as potassium alum (KAl(SO4)2·12H2O). The organic dyes used in red lake pigments range from anthraquinone-based compounds such alizarin and purpurin (extracted from the roots of plants in the madder genus) to xanthene-based scaffolds such as eosin. These pigments have been used by artists for hundreds of years, with notable examples including Claude-Oscar Monet and Vincent van Gogh. The historical importance and prevalence of these pigments in works of art throughout recent history motivate their study; however, detecting and identifying specific red lake pigments using non-invasive techniques remains a challenge in the field of cultural heritage. Not only are these pigments difficult to identify and map in heterogeneous mixtures due to the presence of other (sometime fluorescent) pigments and constituents, but little is also known about their natural ageing and degradation pathways. In this project, we aim to gain a better mechanistic understanding of degradation that occurs in heterogeneous materials containing red lake pigments. Specifically, we use fluorescence spectroscopy to identify conditions under which fluorescence quenching (and presumably degradation) occur and then utilize vibrational spectroscopies such as Raman and FTIR to probe changes to the pigment on a molecular level under these specific conditions.
Dr. Jessica Heimann
Two-Dimensional Layered Nanosheets Synthesis and Various Surface Modifications for Potential Applications
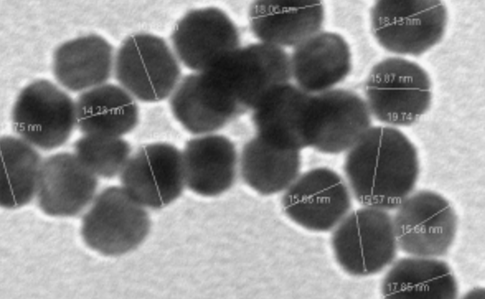
A nanosheet is a layered two-dimensional nanostructure ranging from 1 to 100 nm in thickness and has properties that are completely different from the bulk precursor. Layered materials are defined as solids with strong in-plane chemical bonds but weak out-of-plane van der Waals bonds. They represent a diverse and largely untapped source of two-dimensional systems with exotic electronic, electrochemical, and photonic properties and high specific surface areas that are important for potential applications. Our project (supported by the NSF-Center for Sustainable Nanotechnology) involves synthesis, characterization and surface chemistry modifications of InSe 2D nanosheets and understanding the impact of these surface modified nanosheets on model membranes and organisms relevant to human health and the environment.
Shreyasi Sengupta
Dye-Doped Semiconducting Polymer Nanoparticles for Multiplex Imaging
Multiplex imaging has been a useful tool for biological applications where multiple targets need to be detected and monitored simultaneously. One key weakness of multiplex imaging is that it requires several excitation sources to excite multiple fluorophores. This is especially challenging for near-infrared (NIR) emitting fluorophores, a spectral region where excitation sources are limited. To overcome this limitation, we are working on developing a series of semiconducting polymer nanoparticles (Pdots) doped with chlorins of varying molecular structure. Pdots were synthesized using nanoprecipitation of poly[(9,9-dioctylfluorenyl-2,7-diyl)-alt-co-(1,4-benzo-{2,1’,3}-thiadiazole)] and dye. The size and morphology of the Pdots are measured using dynamic light scattering (DLS) and fluorescence was monitored under excitation of the polymer. The dye doped Pdots are found to have efficient energy transfer from the polymer to the dye, resulting in strong NIR emission when exciting the polymer. Controlling the molecular structure of the chlorin dyes enables tunable emission when excited with a single excitation source.
Connor Riahin
Nanoparticle Interaction Effects on the Membrane Phase of Model Cell Membranes
As they are released into the environment at the end of their product life, nanoparticles may have a variety of adverse effects on organisms. Some of these effects may result from interactions that do not destroy the membrane. One such effect – a change in the phase of a membrane – can affect important cellular functions such as transport and signaling. Laurdan, a phase-sensitive membrane dye, fluoresces blue (about 440 nm) in the solid-ordered phase and a greener blue (about 490 nm) in membranes in the liquid-disordered phase. The phase of the membrane is commonly quantified by inserting the intensities of these two colors from the fluorescence emission spectrum of laurdan into the equation for generalized polarization (GP). In this project, we are measuring the GP of larudan-labeled liposomes (phospholipid vesicles which are simple models of cell membranes) as they are exposed to various nanoparticles made in the Center for Sustainable Nanotechnology [including lithium ion battery cathode materials such as nickel manganese cobalt (NMC) and lithium cobalt oxide (LCO)] to determine their effect on membrane phase. Currently, we are using fluorescence spectroscopy for these measurements and later plan to use microscopy to improve statistical analysis and to correlate effects with specific interactions. From these experiments, we hope to better understand the types of interactions that occur between nanoparticles and membranes and consequently prevent ones which have adverse effects.
Laura Kesner